Prof.Dr Kannan Vishwanatth *
The Chinese University of Hong Kong.
Emil: drkannanvish@yahoo.com
Abstract
A simple and fast flow-injection method for the determination of omeprazole in pharmaceutical preparations is proposed. Detection of the drug is based on the measurement of the absorption signals for the product resulting from the oxidation of omeprazole with N-bromosuccinimide in acidic medium. A flow rate of 0.5 ml/min was pumped and active material was detected at 370 nm. Limit of detection (LOD) and limit of quantitation (LOQ) were calculated to be 2.0 and 6.5 μg/ml, respectively. No interference was observed from common pharmaceutical additives. The method was successfully applied to the assay of omeprazole in capsule preparations and in spiked human plasma. The results were statistically compared with those of the official high-performance liquid chromatography (HPLC) method of the USP by applying Student’s t-test and F-test.
Key Words: Omeprazole, Flow Injection Analysis, Pharmaceutical products, human plasma.

*Corresponding author; e-mail address: Drkannanvish@gmail.com ( Dr Kannan Vishwanatth).
1. Introduction
Omeprazole, 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-pyridinyl) methyl] sulfinyl] -1H– benzimidazole, is a gastric acid pump inhibitor, dose dependently controls gastric acid secretion by altering the activity of H+/ K+ ATPase, which is the final common step of acid secretion in parietal cells [1,2]. It is used for the treatment of acid-peptic diseases such as duodenal, gastric and esophegeal ulceration [2].
Several methods such as HPLC [3,4], liquid chromatography–mass spectrometry [5], voltammetry [6] and spectrophotometry [7-10] have been developed for the determination of omeprazole in pharmaceutical product and biological fluids. The Flow Injection Analysis (FIA) technique has found recently wide applications mainly due to reduction of the analysis time and reagents consumption compared with conventional manual procedures. Our research team has a wide background in the automation of analytical techniques. Several automated techniques for the quantification of several drugs in biological fluid and pharmaceutical products were developed [11-16]. The literature is still lacking a flow injection analysis method for the determination of omeprazole. This paper describes a FIA method for the determination of omeprazole in pharmaceutical products and human plasma.
2. Experimental
2.1. Chemicals
All chemical used were in pure grade and used as received without further purification. The active ingredient omeprazole was supplied by the United Pharmaceuticals. All pharmaceutical products used in this work were purchased from the local market. N-bromosuccinimid was obtained from Fluka. Ultrapure water was used to prepare all solutions. All solutions were prepared daily.
2.2. Instrumentation and analytical conditions
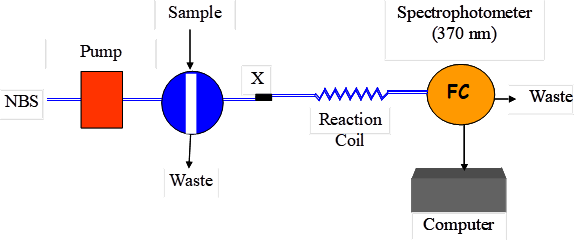
The flow system was assembled using an isocratic HPLC instrument. Simple single line FIA system was used for this purpose (Figure 1). The sample containing the drug under investigation was directly injected into the reagent stream (1.0 mM NBS in 0.2M HCl). The reagent was pumped at a rate of 0.5 ml/min. After injection, the valve was returned to the load position when the maximum change in absorbance value has been reached. The absorbance of the reaction product was monitored at 370 nm.
2.3. Preparation of solutions
a. N-bromosuccinimid (NBS) solution: Accurately weighed 44.5 mg of NBS was transferred to 250 ml volumetric flask and dissolved in 0.1 M HCl. The final concentration was 1.0 mM.
b. Omeprazole standard solutions: Accurately weighed 100.0 mg of omeprazole reference standard was transferred to 100 ml volumetric flask and dissolved in 0.1 M HCl. Standard solutions for linearity study were prepared by diluting the calculated volumes of the stock solution with 0.1 M HCl.
c. Omeprazole capsules: The contents of 10 capsules (20 mg omeprazole/cap) were emptied and finely powdered. The powder amount equivalent to 20.0 mg of omeprazole was transferred to a 100 ml volumetric flask, stirred for 10 min with 0.1 M HCl and then diluted to volume with 0.1 M HCl. The concentration of omeprazole in this solution is 200 ppm. The solution is then filtered through 0.2 µm cellulose acetate syringe filters. The filtrate was used to prepare different concentrations within the linearity range by proper dilution with 0.1 M HCl.
d. Spiked plasma samples: Known amounts of omeprazole were added to drug-free plasma (200 – 600 µl of the 1000 μg/ml per 1.0 ml plasma). The mixture was vigorously mixed and diluted to 5 ml with 0.1 M HCl solution. After that, samples were directly injected without any clean up steps. The recovery following the extraction procedure was determined by comparing the peak height of the spiked plasma samples with those of control samples. The control samples were prepared by mixing solutions containing the same amounts of the drug without plasma.
3. RESULTS AND DISCUSSION
3.1. Development of the experimental conditions
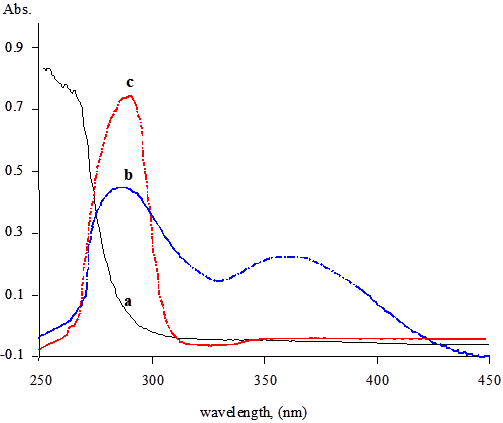
It was found that NBS can oxidize omeprazole in an acidic medium. The absorption spectrum of the oxidation product has a maximum absorption at 370 nm (Figure 2). The absorbance is directly related to the concentration of omeprazole and can be used for its spectrophotometric determination. The development of the product depends very much on the reaction conditions. Oxidation of the drug was found to take place in acidic media. Various acids including acetic, sulphuric, perchloric, nitric, hydrochloric and phosphoric acids were tested. Hydrochloric acid was found to give the highest sensitivity by enhancing the absorbance. Therefore, variable concentrations of NBS prepared in different concentrations of HCl were tested. 0.2 M HCl was found to be adequate. Higher acid concentrations increase the blank readings and reduce the stability of the baseline. Reasonable sensitivity and baseline stability were achieved when 1.0 mM NBS dissolved in 0.2M HCl.
Similarly, FIA variables were also optimized by changing one variable and keeping all others constant. The influence of changing the flow rate and the reaction coil length was investigated. A flow-rate of 0.5 ml/ min was chosen because it gave the highest analytical signals. With a flow rate of 0.5 ml/min, the analysis time for one injection was about 40 seconds and the sample throughput was over 90 samples/h. The length of the reaction coil was varied over the range 20 – 80 cm. A considerable increase in the analytical signal was observed upon increasing the coil length up to 40 cm, and started to decrease after that. Therefore, a 40 cm reaction coil was chosen as the optimum to ensure high sensitivity and a high measurement rate.
3.2. Evaluation of the proposed method
The calibration graph was constructed by injecting standard solutions of omeprazole, in the range of 5 to 200 ppm. The curve was linear up to 120 ppm, with correlation coefficient of 0.9993. A flow-gram obtained under the optimum conditions for a series of standards is shown in Figure 3.
The limit of detection (LOD) was evaluated statistically as the concentration of analyte leading to a signal that is three times the blank standard deviation. Similarly, the limits of quantitation (LOQ) were determined as the concentration of analyte leading to a signal that is ten times the blank standard deviation. The LOD and LOQ were found to be 2.0 and 6.5 μg/ml, respectively indicating a high sensitivity of the method.
The intra-day (within-day) precision was evaluated by replicate analysis of omeprazole within the linearity range at different time intervals. Three measurements were taken within the same day. Each time, a new calibration graph was constructed. The result obtained shows RSD of 0.8% indicating good intra-day precision. Similarly, the inter-day (different days) precision was evaluated on different days (3 days). The results indicated high precision, as the percent RSD was 1.2%. Figure 3 shows the flow signals for the calibration graph and reproducibility test. The reproducibility of the peak height was very good and the relative standard deviation of 10 injections was 1.2% (Figure 3).
3.3. Analysis of real samples
The applicability of the proposed method was checked by analyzing commercially available formulations containing omeprazole. The results are summarized in Table 1. No interferences were observed from the additives normally present in commercially available products and recoveries in the range of 98.8 and 102.9%. The accuracy of the proposed method was investigated by comparing the results obtained with those claimed by the manufacturer and those found by application of the official HPLC method [17]. Six replicate measurements were made in each case; the results obtained were validated by comparison with the HPLC procedure [9] by means of t- and F-tests at 95% confidence level (Table 1). No significant differences were found indicating good accuracy and precision.
The applicability proposed method was further validated using spiked human plasma samples. The results showed excellent recoveries (> 95%) with RSD less than 3.0%, indicating good accuracy and precision. No interferences from the indigenous plasma components were observed. The simplicity of this approach, in which no sample clean up was used especially for biological fluids and the high extraction efficiency are among the essential features of the proposed method, making this method suitable for routine, efficient and fast for the analysis of omeprazole in complex matrices.
4. Conclusions
In conclusion, the proposed FI procedure can be used for the analysis of omeprazole in pharmaceutical products without interference. The method is very simple, using minimum number of reagents and reaction sequence. The speed of analysis and the precision make this method suitable for the quality control of formulations containing omeprazole replacing tedious, expensive, and slow official or chromatographic methods.
Acknowledgment
The support from The Chinese University Hong Kong is gratefully acknowledged. The author would like to thank Dr Ashleys Limited Hong Kong for providing the standard and the excipients.
Table (1): Determination of omeprazole in pharmaceutical preparations
| Pharmaceutical Product | Taken (µg/ml) | Recovery ± RSD, % | |
| Proposed method % ± SD, (n=6) | HPLC % ± SD, (n=6) | ||
| Omedar | 10.0 | 99.5 ± 0.9 | 102.1 ± 2.3 |
| 25.0 | 99.4 ± 0.9 | 100.7 ± 2.7 | |
| 50.0 | 100.4 ± 1.7 | 100.2 ± 1.8 | |
| 100.0 | 101.1 ± 1.9 | 101.4 ± 2.2 | |
| t = 1.13 f = 3.2 | |||
| Oprazole | 10.0 | 102.9 ± 1.8 | 101.3 ± 2.2 |
| 25.0 | 101.7 ± 0.9 | 102.1 ± 1.6 | |
| 50.0 | 98.8 ± 1.1 | 101.2 ± 1.7 | |
| 100.0 | 99.7 ± 1.6 | 100.7 ± 1.1 | |
| t = 1.13 f = 3.2 |
Table (2): Recovery of omeprazole from spiked human plasma samples
| Taken (µg/ml) | Found ± SD (µg/ml) | % Rec. ± RSD |
| 20.0 | 19.4 ± 0.4 | 97.1 ± 2.3 |
| 40.0 | 38.0 ± 1.0 | 95.3 ± 2.6 |
| 60.0 | 58.6 ± 0.6 | 97.7 ± 1.1 |
Figure (1): Schematic diagram of the proposed FIA system. P, pump; X, confluence point; R, 1.0 mM NBS; RC reaction coil; FC, flow cell.
Figure 2: Absorption spectra for pure NBS (a); pure omeprazole (80 µg/ml) (c) and the reaction product of 80 µg/ml omeprazole with NBS (b).
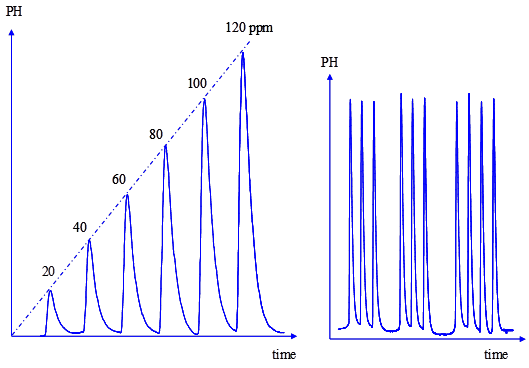
Figure 3: Flow signals for the calibration graph and reproducibility test of omeprazole.
References
[1] Dollery C. (1999), Therapeutic Drugs, published by Churchill Livingstone, Edinburgh.
[2] Burham T. H. (2001). Drug Facts and Comparisons Hebel, S.K., St. Louis. A Wolters Kluwer.
[3] Castro D., Moreno M. A., Torrado S., Lastres J. L., (1999). Comparison of derivative Spectrophotometric and liquid chromatographic methods for the determination of omeprazole in aqueous solutions during stability studies. Journal of Pharmaceutical and Biomedical Analysis 21, 291–298.
[4] Macek J., Ptacek P., Klima J. (1997). Determination of omeprazole in human plasma by high performance liquid chromatography. J. of Chromatography B, 689:239-243.
[5] Wang J., Wang Y., Fawcett J. P., Wang Y., Gu J. (2005). Determination of omeprazole in human plasma by liquid chromatography-electrospray quadrupole linear ion trap mass spectrometry. J Pharm Biomed Anal. 2005 Sep 15;39(3-4):631-5.
[6] Shahrokhian S., Ghalkhani M., Bayat M., Ghorbani-Bidkorbeh F. (2015). Voltammetric behavior and determination of trace amounts of omeprazole using an edge-plane Pyrolytic graphite electrode. Iranian Journal of Pharmaceutical Research (2015), 14 (2): 465-471
[7] Al-Momani I. F., Rababah M. H. (2010). Validation of HPLC and FIA spectrophotometric methods for the determination of lansoprazole in pharmaceutical dosage forms and human plasma. American Journal of Analytical Chemistry, 2010, 1, 34-39
[8] Kumaraswamy D., Stephenrathinaraj B., Rajveer C. H., Sudharshini S., Shrestha B., Rao R. (2010). Process validation of analytical method development and validation for omeprazole capsules and blend. International Journal of Pharma and Bio Sciences, 1(2), 1-6.
[9] Al-Momani I. F. (2001). Spectrophotometric determination of selected cephalosporins in drug formulations using flow injection analysis Journal of Pharmaceutical and Biomedical Analysis, 25, 751–757.
[10] Klyankar T., Wadher S. J., Gholve R., Anitha K. (2016). UV Spectrophotometric estimation of diclofenac potassium and omeprazole magnesium in bulk and combined tablet dosage form. International Journal of MediPharm Research, 2(1), 42-53.
[11] Syed A. A., Syeda A. (2008). Spectrophotometric determination of certain benzimidazole proton pump inhibitors, Indian J. Pharm. Sci., 2008, 70 (4): 507-510.
[12] Al-Momani I. F. (2004). Flow-Injection spectrophotometric determination of amoxcillin, cephalexin, ampicillin, and cephradine in pharmaceutical formulations. Analytical Letters, 37(10), 2099–2110.
[13] Al-Momani I. F. (2006). Flow Injection spectrophotometric determination of the antibacterial levofloxacin in tablets and human urine. Analytical Letters, 39: 741–750.
[14] Al-Momani I. F. (2006). Indirect Flow-Injection spectrophotometric determination of meloxicam, tenoxicam and piroxicam in pharmaceutical formulations. Analytical Sciences, 22, 1611-1614
[15] Al-Momani I. F., Kanan S. J. (2008). Flow-Injection spectrophotometric and LC determination of doxycycline, oxytetracycline and chlortetracycline in biological fluids and pharmaceutical preparations. Flow Injection Analysis 25(1), 29–34.
[16] Al-Momani I. F., Haj-Hussein A. T., Tahtamouni A. N., J. )2008). Flow injection spectrophotometric and chromatographic determination of ciprofloxacin and norfloxacin in pharmaceutical formulations. Flow Injection Analysis 25(2), 151–155
[17] United States Pharmacopeia 24, United States Pharmacopeia Convention, Asian Edition, the Board of Trustess, 2000, p. 1217.
Armed with Chemical Engineering degree, Dr Kannan Vishwanatth is currently the promoter Director of Hong Kong-based pharmaceutical company Rupus Global Limited.Dr Kannan Vishwanatth is a global opinion maker of contemporary issues & a much sought after speaker in various international forums. Dr Kannan is credited with reputation for innovation, social connections, track record for value creation and investor expectations for value creation. As a Research Scholar, Dr. Kannan has published many research papers & is associated with many top notch International Institutions as Editorial Reviewer. Dr. Kannan Vishwanatth is a global Citizen & a strong believer in Corporate Social Responsibilities. Over the years, Dr. Kannan has slowly transitioned away from Corporate World and into philanthropic & academic ventures. Dr. Kannan Vishwanatth, 45 years, is the Founder and Promoter & Managing Director of our Company. He holds a doctorate in Business Management (Ph.D.). He has an experience of 20 years in the pharmaceutical industry. As the Promoter & Managing Director, Dr. Vishwanatth, has been the backbone of our Company’s operations and is involved in formulating the Company’s strategy. Under his guidance, our Company, ventured into new geographies with a wide product range in various therapeutic segments. His vision and value system have guided the organization towards profitable sustainability. Believing in delegation of responsibility, Dr. Vishwanatth created a professional team and expects Rupus Global Limited to emerge as a global player across multiple therapeutic segments.